![]()
My Yeast Let Me Down: A Love Song
Research Program
Our current studies focus on cellular and molecular investigation of HIV-1 viral protein R (Vpr). Specifically we are interested in understanding host responses to vpr gene expression and the specific role of Vpr in cell cycle G2/M regulation, viral pathogenesis, and anti-cancer gene therapy. Most recently, we have started to study molecular mechanisms of drug resistance of HIV-1 proteases. High throughput screening systems have been established to screen drugs against HIV-1 proteases and Vpr.
During
infection of host cells by HIV-1, active host-pathogen interactions take place.
The final balance between these interactions determines the efficiency of viral
infection and subsequent disease progression. HIV-infected
cells respond to viral invasion with various antiviral strategies such as
innate, cellular and humoral immune antiviral defense mechanisms. On the other
hand, the virus has also developed tactics to suppress these host cellular
responses. Among the many viral offensive strategies, viral protein R (Vpr)
plays a particularly active role. It is involved in nuclear transport of the
viral pre-integration complex, activation of viral transcription, induction of
cell cycle G2/M arrest and apoptosis of the host cells. However, specific roles
of these Vpr activities in viral pathogenesis and their contribution to disease
progression are not fully understood. HIV-1
defective for some or all of these Vpr activities have been associated with slow
disease progression in some patients. With regard to the host responses to vpr
gene expression, studies show that Vpr is specifically targeted by CD8
T-lymphocytes during acute viral infection and that the host innate immune
response may also play a crucial role in suppressing the effects of Vpr on
various cellular activities. The effect of host cellular responses to vpr
gene expression and its roles in nuclear transport, cell cycle G2/M regulation
and induction of apoptosis are subjects of our studies. For recent review, see
Zhao,
Bukrinsky and Elder, 2005.
The cell cycle is an
orderly process that ensures faithful replication and distribution of all genes
to the next generation, and there are controls on the cell cycle which help to
protect the integrity of the genome.
One of these important control systems is a cellular surveillance process
known as the cell cycle G2/M checkpoint.
When DNA is damaged or DNA synthesis is inhibited, progression through
the cell cycle is halted at the G2/M boundary by the DNA damage or DNA
replication checkpoint.
The G2 arrest or delay induced by these checkpoint control systems is
believed to allow sufficient time to repair DNA damage or completion of DNA
synthesis prior to entering mitosis.
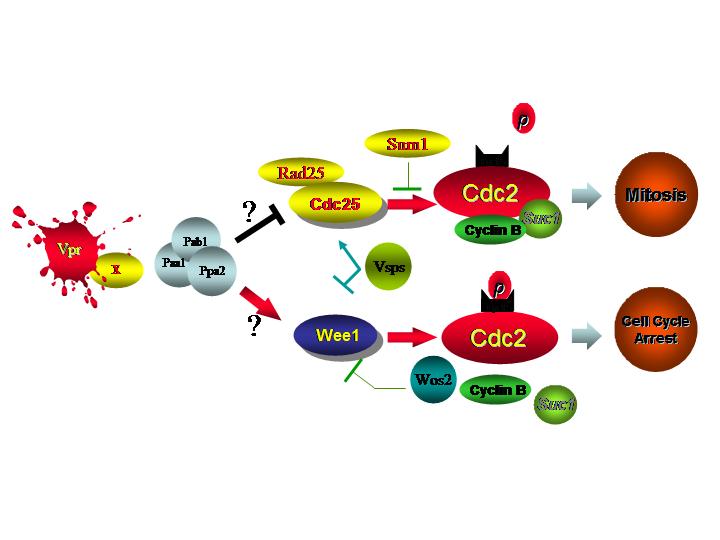
The G2/M transition in eukaryotes is normally regulated by the synergistic and opposing activities of a cascade of distinct protein kinases and phosphatases. This cascade converges on Cdc2, a cyclin-dependent kinase Cdc2, which determines the onset of mitosis in all eukaryotic cells. In fission yeast, entry into mitosis is regulated by the phosphorylation status of Tyr15 on Cdc2, which is phosphorylated by Wee1 and Mik1 kinases during G2 and rapidly dephosphorylated by the Cdc25 phosphatase to trigger entry into mitosis.
 |
|
| Model 1: The classic mitotic G2/M checkpoint controls | Model 2: A putative cell cycle G2/M mechanism modulated by HIV-1 Vpr |
These mitotic regulators (Wee1, Mik1 and Cdc25)
are the targets of two well-characterized DNA damage and DNA replication
checkpoint pathways which induce cell cycle G2 arrest (Model
1. Click the picture for a larger view). The DNA damage checkpoint activated by radiation leads to inhibitory
phosphorylation of Cdc2 by a pathway, which appears to be highly conserved
between fission yeast and mammalian cells. In fission yeast the early
genes in the pathway, which include Rad1, Rad3, Rad9, Rad17 and Rad26 are
thought to detect the DNA damage and lead to the phosphorylation of the Chk1
protein. The activated Chk1 kinase
then directly phosphorylates the Cdc25 phosphatase. The phosphorylated Cdc25 binds the Rad24/25 proteins, and this complex
is transported out of the nucleus to render Cdc25 inactive. Both Rad24 and Rad25 are homologues of a human 14-3-3 protein, which
has a similar Cdc25 inhibitory function. Upon DNA damage Chk1 also activates the
Mik1 kinase to inhibit Cdc2. DNA damage thus initiates a protein phosphorylation cascade ending in
the inactivation of the Cdc25 phosphorylase and activation of Mik1 kinase to
increase inhibitory phosphorylation of Tyr15 on Cdc2.
Inhibition of DNA replication by
chemical agent such as hydroxyurea in fission yeast also leads to cell cycle
arrest through inhibitory phosphorylation of Cdc2
(Model 1), and this DNA replication checkpoint pathway again appears to be highly
conserved in mammalian cells. Parts of this DNA replication checkpoint are shared with the DNA damage
checkpoint as Rad1, Rad3, Rad9, Rad17, and Rad26 are required for both
checkpoints in fission yeast. However, the DNA replication checkpoint acts primarily through
phosphorylation of the Cds1 kinase with minor participation of the Chk1 kinase,
and either kinase is sufficient by itself to give cell cycle arrest when DNA
synthesis is inhibited. The activated Cds1 kinase inactivates Cdc25 through the same mechanism
as Chk1 and may also activate Wee1 and Mik1 kinases, which phosphorylate Tyr15
of Cdc2.
HIV-1
Vpr also arrests human and
fission yeast cells in the G2 phase of the cell cycle by increasing the
phosphorylation of Tyr15 on the Cdc2 (Cdk1), a typical response of the cellular
G2/M checkpoint control (Model 2). However, the two well-defined mitotic checkpoint
control pathways are not exclusively responsible for the G2 arrest induced by
Vpr. We have demonstrated that Vpr uses an alternative cellular
pathway to regulate the G2/M transition, which include PP2A, Wee1 and Cdc25. A
number of protein candidates that bind to Vpr have been found. Genetic and
multiple-copy suppressors of Vpr were identified. Their functional relevance to
induction of G2 arrest is being characterized.
The
Role of Vpr in Nuclear Transport of Viral Pre-integration Complex 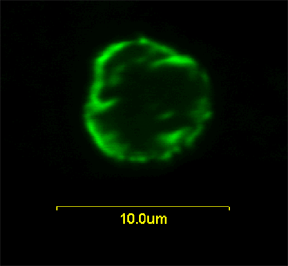
HIV-1
Vpr plays a pivotal role in the
nuclear transport of the viral pre-integration complex (PIC). However, the
precise molecular mechanism underlying this process is controversial. Most
studies have reported that Vpr expressed without other viral proteins localizes
predominantly to the nuclear envelope in human, fission yeast (right picture) and budding yeast.
Increasing evidence suggests that nuclear
localization of Vpr may use a modification of the importin a
pathway. However, it
was initially suggested from in vitro
assays that Vpr did not use the importin a pathway since addition of excess cNLS did not inhibit the nuclear
localization of Vpr. Further studies
showed that Vpr does bind to importin a
both from human and budding yeast, but the binding site is different from the
binding site for cNLS (see Model,
click for a larger view). Vpr also binds to
nucleoporins, and based on this interaction suggested that Vpr might function directly as a receptor for nuclear
transport in place of importin b. Alternatively, importin b
might be necessary for nuclear localization of Vpr and that importin a, importin
b, cNLS and Vpr form a ternary complex.
Thus, the Model best supported
by the evidence at present is that Vpr binds to a previously unknown site on
importin a, and this complex in turn binds to importin b which serves as
the receptor for transport through the nuclear pore.
The effect of Ran-GTP binding to importin b
on the ternary complex has not been reported and it is not understood why Vpr is
frequently observed to be at the nuclear envelope although this may be related
to the binding of Vpr to nucleoporins. One study found Vpr to be
at the inside of the nuclear envelope suggesting that Vpr is transported through
the pore but then stops at the inside of the nuclear pore rather than being
released into the nucleus.
Another study showed that Vpr causes nuclear herniation which ruptures nuclear
membrane thus enhancing the chance for entry of PIC into the nucleus. For
reviews, see (Zhao
and Elder, 2000; Zhao et al., 2004).
The Role of Vpr in Viral Pathogenesis
Vpr causes severe cellular dysfunctions that are lethal, which could contribute to the CD4+ T-cell depletion observed in HIV-infected patients. Understanding the mechanisms by which Vpr affects host cellular functions will provide new insights into HIV pathogenesis and is crucial for developing new strategies to block the cytopathic effects of Vpr. However, as Vpr displays multiple activities in the infected cells, a critical question is whether all of these activities are of equal importance to viral pathogenesis. We have developed and validated a rapid and efficient fission yeast system to test the multiple Vpr functions simultaneously. This rapid Vpr functional screening system provides an unique opportunity for us to directly address the relevance of Vpr functions to pathogenesis in HIV-infected patients.
In
collaboration with Dr. Nitin Saksena’s laboratory in
Vpr as a Potential Natural Biological Agent for Cancer Therapy
The unique ability of Vpr to arrest the cell cycle in G2 provides an opportunity to explore its potential as a cell cycle "G2 blocker" to inhibit growth of the cancerous cells. Furthermore, since mammalian cells residing in the G2 phase of the cell cycle are most sensitive to radiation, Vpr-induced G2 arrest may sensitize tumor cells to radiation. The combination of gene and radiotherapies based on Vpr and the radiosensitivity of G2 cells may lead to the development of new methods to treat cancer.